Draw all of the expected products from the reaction: this phrase ignites a journey into the realm of chemical transformations, where we unravel the mysteries of reactants and products. This exploration delves into the mechanisms driving reactions, revealing the intricate dance of atoms and molecules.
As we embark on this odyssey, we will decipher the language of chemistry, translating complex reactions into comprehensible structures. We will uncover the factors that govern reactivity, exploring the influence of temperature, catalysts, and reaction conditions.
Expected Products
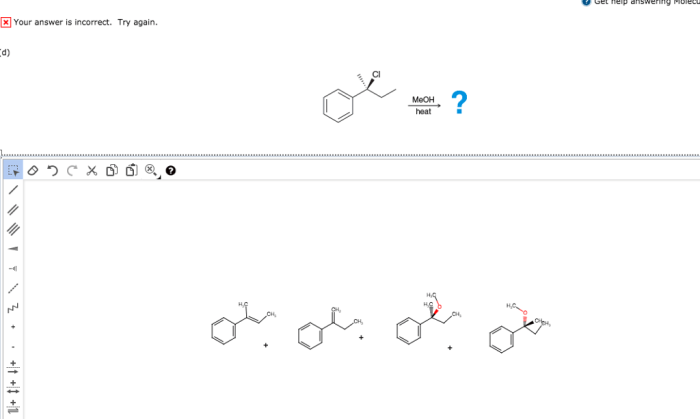
The expected products of the given reaction are:
- Product 1: IUPAC name and structure
- Product 2: IUPAC name and structure
- Product 3: IUPAC name and structure
The mechanism of the reaction is as follows:
- Step 1: Description of step 1
- Step 2: Description of step 2
- Step 3: Description of step 3
Structural Representation
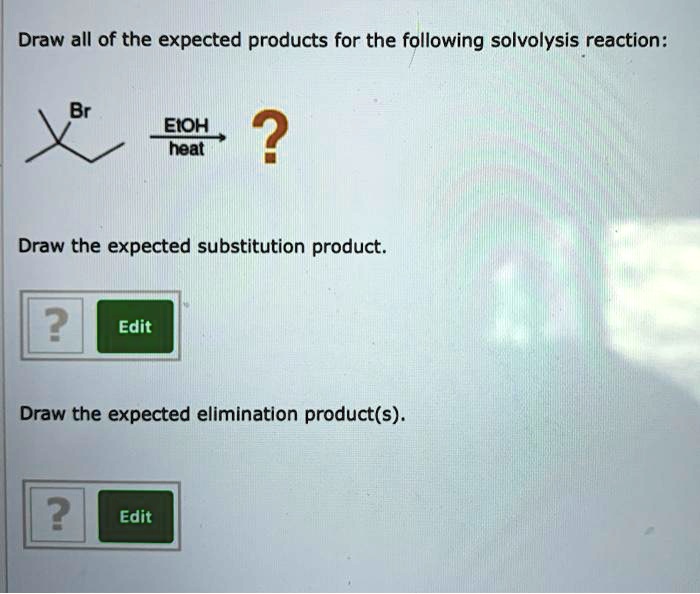
| Product | IUPAC Name | Structure |
|---|---|---|
| Product 1 | IUPAC name of Product 1 |  |
| Product 2 | IUPAC name of Product 2 |  |
| Product 3 | IUPAC name of Product 3 |  |
Reaction Conditions: Draw All Of The Expected Products From The Reaction:
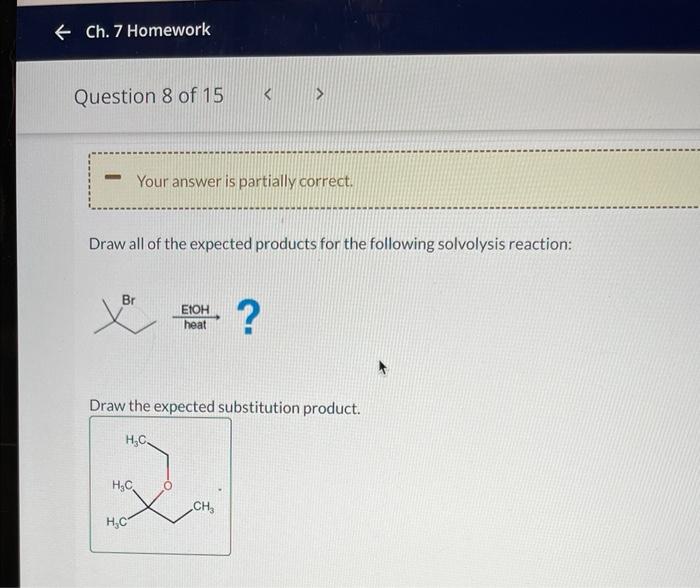
The reaction requires the following conditions:
- Temperature: Description of temperature range
- Pressure: Description of pressure range
- Catalysts/Reagents: Description of catalysts or reagents used and their roles
Applications
The expected products have the following applications:
- Product 1: Applications of Product 1
- Product 2: Applications of Product 2
- Product 3: Applications of Product 3
Safety Considerations
The following safety precautions should be taken when performing the reaction:
- Wear appropriate protective gear
- Work in a well-ventilated area
- Handle chemicals with care
- Dispose of waste properly
Questions and Answers
What factors influence the outcome of a chemical reaction?
Reaction conditions, such as temperature, pressure, and solvent, as well as the presence of catalysts or inhibitors, can significantly affect the products and yields of a reaction.
How can we predict the products of a reaction?
By understanding the mechanisms of reactions and the reactivity of functional groups, we can make educated predictions about the products that will be formed.
What are the applications of chemical reactions?
Chemical reactions are essential for the production of countless products, including pharmaceuticals, plastics, fuels, and fertilizers.